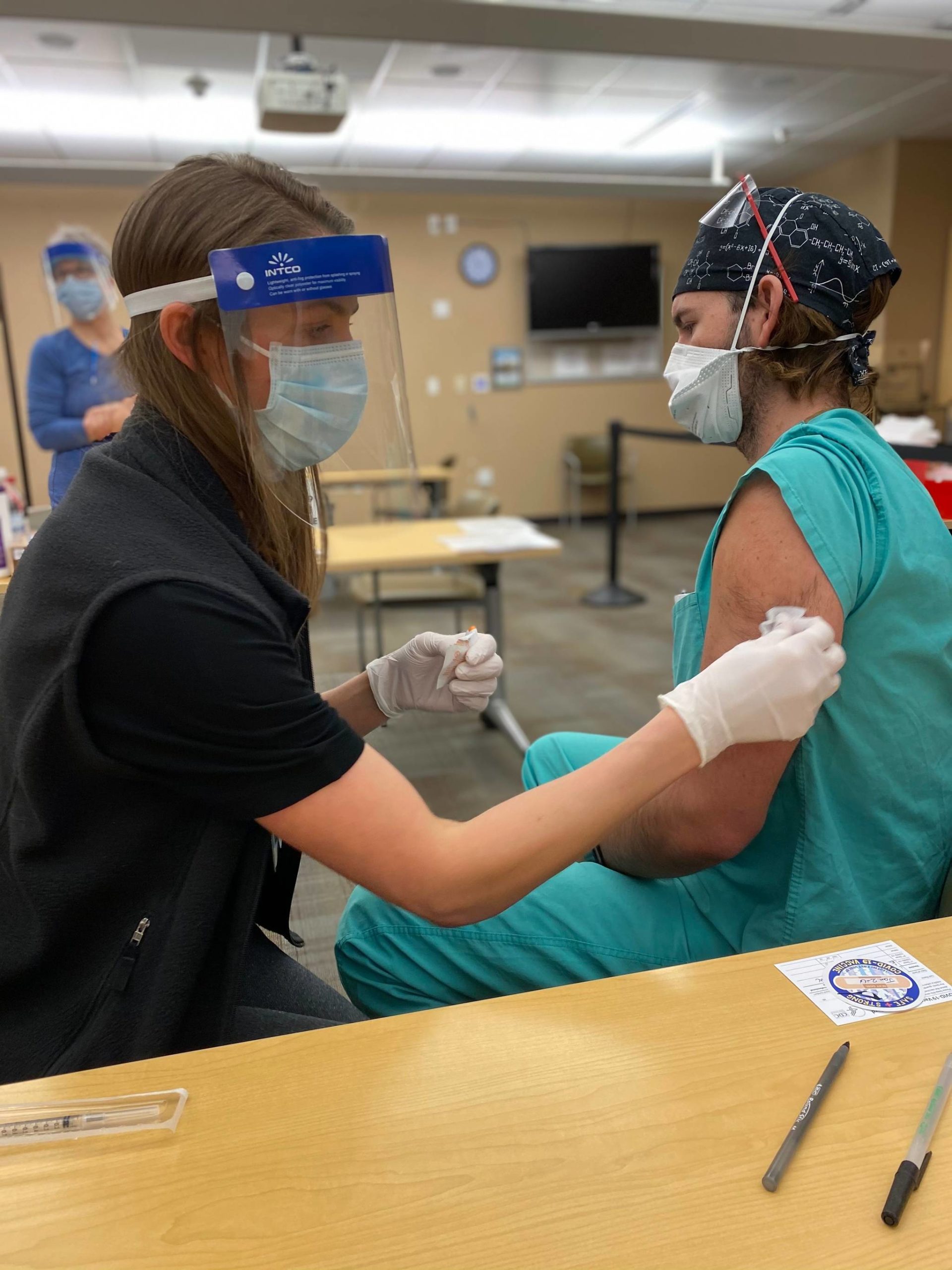
Reported adverse reactions to COVID-19 vaccines should be considered in a broader context, said Alaska’s chief medical officer Dr. Anne Zink.
Zink, speaking during a video news briefing Monday, said clinicians are being encouraged to report all reactions — even extremely mild reactions — and emphasized many adverse reactions to vaccine placebo were reported and that it’s common for people to have negative reactions to routine medical procedures.
“My husband passes out if he gets an IV,” Zink said. “He probably didn’t want me to share that publicly, but that’s what happens.”
She said as more adverse reactions to COVID-19 vaccines are reported —and Zink predicted many more will be —she hopes the reactions are considered in a larger, clinical context.
“This is really common,” Zink said. “We don’t report every time someone passes out when they get an IV in the emergency department because we know that’s a known effect, and we kind of watch throughout the process. But, because this is a new vaccine, we’d rather it be reported, and then we work through that process right now.”
[Multiple adverse reactions to new vaccine reported by Bartlett Regional Hospital]
Zink said there is no reason to think that the adverse reactions that have already been reported, including the nation’s first severe adverse reaction at Bartlett Regional Hospital, were caused by transporting the Pfizer/BioNTech vaccine, which must be kept at extremely cold temperatures. A package containing 20 vaccine doses did; however arrive in Ketchikan too warm to be used, said Matt Bobo, a state epidemiologist for the Division of Public Health.
“But I will tell you, everything is on the table and lots of topics are being discussed,” Zink said.
She did not have an exact number of adverse reactions that have so far been reported, but two instances of anaphylaxis — one in Juneau and one in Fairbanks — have been reported. Tessa Walker Linderman, Department of Health and Social Services lead for the Alaska COVID-19 Vaccination Task Force, said two mild reactions were reported for Providence Alaska Medical Center and a second mild reaction was reported for Bartlett Regional Hospital.
As of Sunday 5,674 COVID-19 Pfizer vaccinations had been given, according to state data.
Bob Onders, interim administrator at Alaska Native Medical Center and medical director for Alaska Native Tribal Health Consortium, said as vaccine doses make their way to remote portions of the state, all 180 village clinics with community health aids are equipped to treat adverse reactions.
“The structure is in place for them to be prepared for this,” Onders said. “They have the resources there. They’ve had them there for years and years.”
Additionally, Zink said the adverse reactions reported happened within the 15-to-30-minute observation period included in federal Centers for Disease Control and Prevention guidance.
“So they’re able to watch them for a sufficient period of time before they leave,” Zink said.
More in store
Doses of the Moderna vaccine are arriving in the state this week, health officials said, with some facilities already receiving shipments of the second COVID-19 vaccine to be approved for emergency use in the U.S.
Walker Linderman said 26,800 doses are allocated for Alaska.
Additionally, the state has defined who will be part of the third tier of the first phase of administering vaccinations in Alaska.
The third tier was created using input from the Alaska Vaccine Allocation Advisory Committee.
To qualify, people must have direct contact with patients or infectious materials from patients; provide essential service in a health care setting that cannot be offered remotely or performed via telework; and provide a service in a health care setting that cannot be postponed with a negative effect on a patient’s health.
Walker Linderman said that tier is expected to start Jan. 4.
• Contact Ben Hohenstatt at (907)308-4895 or bhohenstatt@juneauempire.com. Follow him on Twitter at @BenHohenstatt.